
- Industry news
Industry news
- Category news
Category news
- Reports
- Key trends
- Multimedia
- Journal
- Events
- Suppliers
- Home
- Industry news
Industry news
- Category news
Category news
- Reports
- Key trends
- Multimedia
- Events
- Suppliers
Cultivated beef breakthrough: Scientists overcome cost and technical barriers
Key takeaways
- Scientists discover a method to naturally immortalize cow cells without genetic modification, providing a safe and scalable source for cultivated beef production.
- The study removes major technical and regulatory barriers by demonstrating a natural, non-GM process for cell renewal.
- The discovery could revolutionize sustainable meat production and reduce the environmental impact of beef farming.
Scientists in Israel have developed a method to naturally “immortalize” cow cells without genetic modification or abnormal transformation — challenging long-held beliefs that genetic methods are the only way bovine cells can renew themselves.
The discovery addresses one of the most stubborn bottlenecks in cultivated beef and lamb production by providing a “safe, stable, and scalable” source of cells for sustainable and ethical meat production without the environmental toll of traditional livestock farming.
While similar self-renewal has been achieved previously in chicken cells, this study overturns previous assumptions that such processes were not possible in large mammals due to their natural resistance to cellular transformation, says the team at the Hebrew University of Jerusalem and Believer Meats, who jointly conducted the research.
The study’s lead, professor Yaakov Nahmias from the Grass Center for Bioengineering at the university founded Believer Meats in 2018, which partnered with GEA last year to enhance the scalability and sustainability of cultivated chicken.
Nahmias tells Food Ingredients First that the team has long been focused on developing safe, cost-efficient platforms for cultivated meat production.
“We identified new immortalization pathways in chickens back in 2022, leading us to believe that similar unconventional pathways exist in mammals as well. Sometimes faith is important in science.”
The process, published in Nature, was driven by the activation of telomerase and PGC1α, allowing the cells to “reset their biological clocks” and regenerate mitochondria.
Telomerase is an enzyme that protects the DNA in cells to extend their life, while PGC1α is a protein that regulates the creation and function of new mitochondria, the “energy powerhouses” inside cells.
 Stable, self-renewing bovine cell lines are the foundation for scaling cultivated beef, says author and study lead Yaakov Nahmias.
Stable, self-renewing bovine cell lines are the foundation for scaling cultivated beef, says author and study lead Yaakov Nahmias.
Pushing cell renewal limits
The study highlights that in traditional cell biology animal cells stop dividing after a certain number of generations and enter a stage called “senescence.” Until now, cattle cells could only evade this limit through gene editing, which raised concerns around safety and regulatory approval.
“Ruminant cells are evolutionarily wired to resist immortalization. The hurdle [during the study] was time,” Nahmias shares.
The scientists isolated cells from Holstein and Simmental cows and cultured them in the laboratory for over 500 days. The team tracked the cells’ progression through aging and senescence, “until cell colonies appeared,” he adds.
After 240 cell generations, they observed “spontaneously renewing” bovine cells, which had never been demonstrated before in large mammals.
No disruption in the normal growth regulation of the cells was observed during molecular analysis, and the cells retained their DNA repair capabilities, which the authors note depicts a “natural, controlled pathway of renewal.”
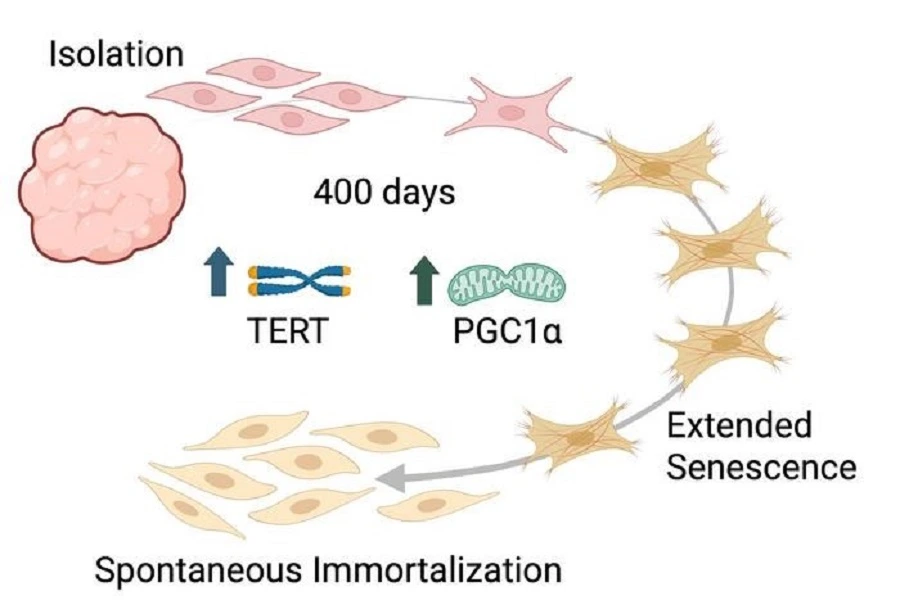 After isolating cow cells, the team waited for over 400 days before colonies “suddenly appeared,” overturning previous beliefs about bovine cells (Image credit: Nahmias Lab).
After isolating cow cells, the team waited for over 400 days before colonies “suddenly appeared,” overturning previous beliefs about bovine cells (Image credit: Nahmias Lab).
Overcoming regulatory barriers
The findings eliminate one of the biggest technical and regulatory barriers to producing affordable cultivated beef, note the researchers. Securing approval across different countries is complex and costly, with manufacturers also facing various bottlenecks around cell line sourcing and culture media.
Nahmias emphasizes that “stable, self-renewing bovine cell lines” are the cornerstone for scaling cultivated beef. Growing the cells without genetic modification “removes a major technical and regulatory barrier” in the field.
“We avoid transformation-associated risks that are common in gene-edited lines because the cells were immortalized naturally, without TP53 mutation or inactivation, and remained karyotypically stable while failing to form colonies in soft agar,” explains Nahmias.
The new method offers a “cleaner starting point” for regulators and consumers, he adds.
Addressing cost and sustainability concerns
The authors note that beef production is the most resource-intensive form of agriculture, responsible for deforestation, water depletion, and global GHG emissions. Cultivated meat is often presented as a solution since it uses animal cells rather than livestock. Its sustainability and animal welfare benefits drive consumer acceptance.
However, challenges associated with cost and safety have “slowed progress,” especially for cultivated beef.
While the economics of cultivated beef production using the study’s new lines look promising, Nahmias stresses that the “true cost depends on process engineering and market conditions validated in commercial systems.”
Earlier this year, food tech firm Ever After Foods collaborated with Bühler to slash cultivated meat production costs by over 90%.
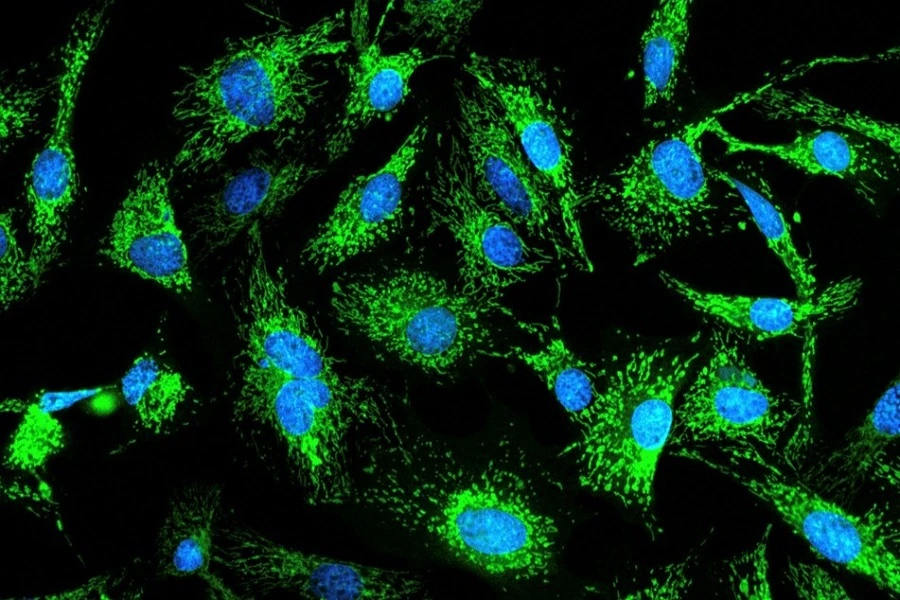 Fluorescent microscopy of immortalized beef cells with the mitochondria labeled green (Image credit: Nahmias Lab).
Fluorescent microscopy of immortalized beef cells with the mitochondria labeled green (Image credit: Nahmias Lab).
Exploring further applications
The technology is “exclusively licensed” to Believer Meats, which recently received USDA approval for its large-scale production facility in North Carolina, US.
“The company is led by an outstanding team of industry experts who are well-positioned to bring this technology to market,” says Nahmias.
Looking ahead, the team plans to investigate whether the natural cell renewal process can be applied to other mammals and whether these cells can be developed into muscle and fat tissues suitable for cultivated meat production.











